Discover the properties and industrial applications of cristobalite
The synthesis of cristobalite
Cristobalite, a high-temperature modification of silicon dioxide (SiO2), is rarely found in nature. For this reason, it is synthesized for industrial purposes by calcining pure silica in a rotary kiln at around 1,500 °C. This process expands the lattice structure, reduces density, and creates air inclusions, resulting in a negative refractive index and a high degree of whiteness. Cristobalite, like silica, is chemically inert and thus valued and used as a filler in various applications and products.

Modifications of silicon dioxide (SiO02) in the oxide mineral group
The modifications include the following minerals:
- Silica
- Cristobalite
- Fused Silica
Although all three share the chemical composition SiO2, they are different in terms of production, certain technical parameters, and fields of application.
Advantages of the surface treatment of Cristobalite
When cristobalite powders are surface treated, numerous “concrete” advantages for industrial use are realized:
- improved resistance to hot water
- high weather resistance
- increased chemical resistance
- improved mechanical strengths
- excellent workability
- consistent and brilliant colors
- higher filling degree
Technical properties
- hardness 6.5 (Mohs)
- density 2.35 g/cm³
- high chemical resistance
- thermal expansion coefficient: 54*10⁻⁶/K (at T 20 – 300°C)
- high degree of whiteness (value Y >94)
Cristobalite from HPF: Products of the highest quality
The products distributed by HPF bear the following brands:
SIKRON®
represents fine cristobalite powders
SILBOND®
offers surface treated cristobalite powders
SILMIKRON®
ultrafine cristobalite powders in untreated and treated form
Cristobalite is synonymous with pure white
Through the calcination process of silica sand in the rotary kiln, a fractured grain surface is achieved. This reduces the density to 2.35 g/cm³. The resulting air inclusions cause a negative refractive index and a very high degree of whiteness. In dental applications, pure white cristobalite allows for excellent coloring and the desired elasticity or Shore hardness in impression materials.

View into the rotary kiln
Surface treatment of fillers: advantages of surface treatment
Various silanes have proven effective in the surface treatment of fillers. A significant advantage of direct surface treatment is that condensation byproducts can escape during the mineral coating. Unlike subsequent in situ surface treatment, direct surface treatment leaves no byproducts in the polymer system. The use of coated fillers facilitates incorporation into a polymer compared to uncoated ones. To ensure optimal bonding between the polymer and high-performance filler, a coating agent specifically adapted to the polymer system is required. This adaptation not only ensures better integration but also optimal performance of the entire composite material.
Our cristobalite – an important raw material for polymer applications
Cristobalite in paints and coatings
- Exterior dispersion-based paints and coatings: the excellent degree of whiteness allows for the production of brilliant colors.
- Road markings: offer a high degree of whiteness and abrasion resistance.
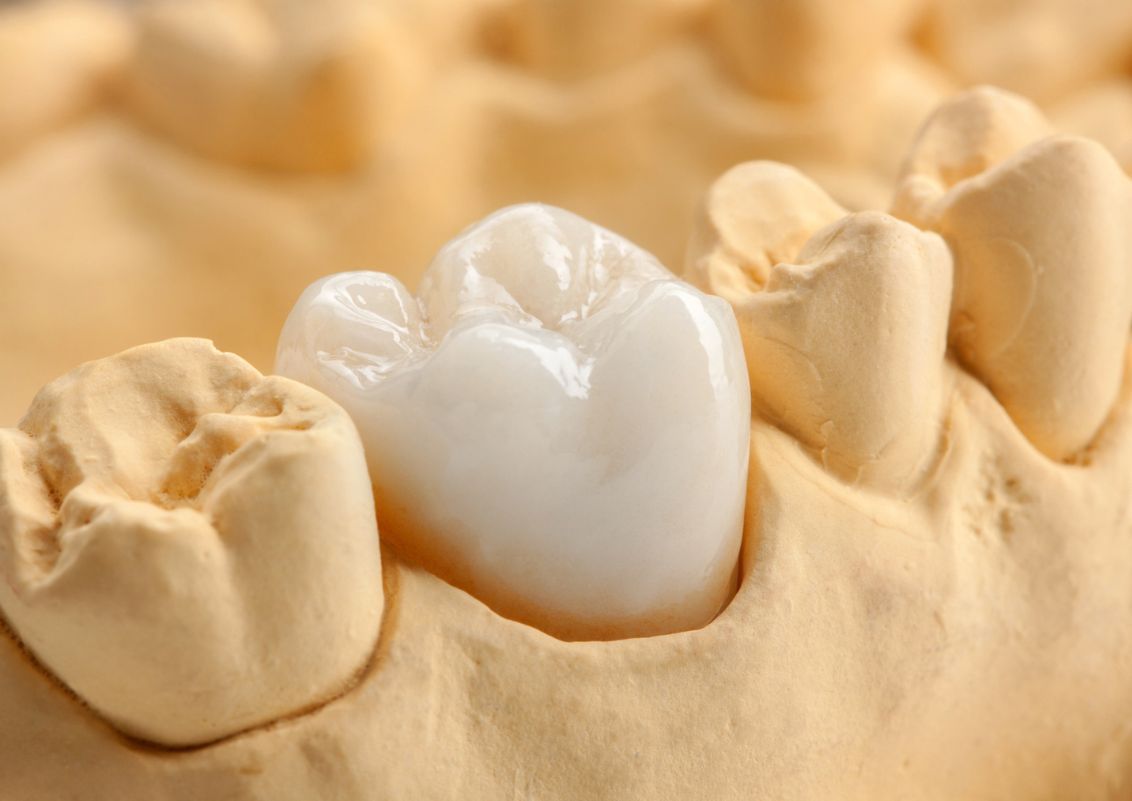
Cristobalite for dental impression compounds
Due to the consistently high quality, cristobalite can also be used in dental products. Below are the advantages of fine and ultrafine cristobalite powders for dental applications summarized:
- high degree of whiteness
- gentle and non-abrasive processing
- narrow particle size distribution/classification, free of coarse grains
- microbiologically safe
- specially selected coating available
Engineered Stone
This is the globally recognized name for artificial stone. These slabs mimic the appearance of natural stone but are made with polymer (plastic) and mineral fillers. Typical applications include countertops and kitchen sinks. The advantages of this material lie in its high hardness, which allows efficient grinding.
Anti-block additives in PP and PE films
Anti-block additives, specifically cristobalite flour, play a crucial role in polypropylene (PP) and polyethylene (PE) films. These plastic films, used in various industries such as food packaging, vehicle construction, medical technology, construction and agriculture must meet different requirements. In addition to mechanical properties, optical criteria such as transparency, gloss, and opacity are also important. The use of mineral fillers such as cristobalite flour offers numerous advantages. An exceptional benefit is the function as an anti-block additive, which facilitates the separation of film sides and prevents unwanted adhesion.
Silicone polymers
Reinforced with SIKRON® and SILBOND®, they stand out through:
- adjustable elasticity
- excellent tear resistance
- high tear strength
- increased modulus of elasticity
- improved electrical insulation properties
- adjustable thermal expansion
- reduced reaction shrinkage
- excellent color ability
Key characteristics of cristobalite
The specific properties make cristobalite a versatile and sought-after material in various industrial applications.
1. Crystal structure: Cristobalite has a different structure compared to the more common silica. It is a high-temperature modification of silicon dioxide (SiO₂).
2. Occurrence: In nature, cristobalite is rather rare and is often found in volcanic rocks. For industrial purposes, it is mainly produced synthetically.
3. Production: Synthetic production is achieved through the calcination of pure quartz at around 1,500 °C. This process expands the lattice structure and reduces density, giving specific properties.
4. Optical properties: Thanks to the production process, cristobalite exhibits air inclusions that lead to a negative refractive index and a high degree of whiteness.
5. Usage: Cristobalite is used in various sectors, including the production of ceramics, enamels, refractory materials, as well as in plastics, paints, varnishes, rubber, and silicone for industry.
6. Viscosity properties: High-performance cristobalite-based fillers offer good viscosity adjustability at high filling levels.
Occurrence and extraction of cristobalite
Cristobalite is found worldwide in various geological formations, and this mineral has also been discovered on the Moon and Mars. Known locations include volcanic areas, particularly in countries with active or historical volcanic activity. Examples of these countries are Italy, Mexico, Indonesia, and the United States. A total of 372 known sites.
In Germany, there are sources of cristobalite, especially in regions with a volcanic past. One known location is, for example, the Eifel region, where deposits of volcanic rocks may contain cristobalite.
The history of cristobalite
Cristobalite was first described in 1884 by Gerhard vom Rath. The name "Cristobalite" derives from the nearby town of San Cristóbal in Mexico, where researchers discovered the mineral. In the 1920s and 1930s, cristobalite began to play a role in various industrial applications; the first determination of its structure by X-ray diffraction occurred in 1925. Particularly in the ceramics and glass industry, cristobalite was appreciated for its special properties.
Today, cristobalite is used in various industrial sectors, such as the production of plastics, ceramics, enamels, and refractory materials. Even in the modern construction materials industry and the production of silicone products, companies use cristobalite. Advances in processing technology have helped expand the application possibilities of cristobalite. Surface modification of cristobalite allows specific adaptations for various industrial needs.
FAQ
What properties does cristobalite have?
Cristobalite is a high-temperature modification of silicon dioxide (SiO₂) with a tetragonal crystal structure. It features a high degree of whiteness, a hardness of about 6.5 on the Mohs scale, and a low density of about 2.35 g/cm³. Additionally, it exhibits high chemical resistance and an increased thermal expansion coefficient of 54 × 10⁻⁶ K⁻¹ in the temperature range from 20 °C to 300 °C.
What is cristobalite used for?
Cristobalite is used in various industries. In the paint and coatings industry, it serves as a filler in exterior dispersion-based paints and plasters to enhance whiteness and resistance. In dental technology, it is used in impression compounds due to its high purity and microbiological safety. Additionally, it is used in the production of Engineered Stone to improve the hardness of products. In the plastics industry, cristobalite acts as an anti-block additive in polypropylene and polyethylene films to prevent adhesion of film layers. Other applications include silicone sealants and road markings.
How does cristobalite work?
The effectiveness of Cristobalite in these applications is based on its high chemical resistance, high degree of whiteness, and specific particle size structure. In paints and coatings, it improves coverage and weather resistance. In dental materials, it ensures high precision and biocompatibility. In artificial stones, it increases mechanical strength and durability. As an anti-block additive, it reduces adhesion between plastic layers, facilitating film handling.
How is cristobalite produced?
Cristobalite is industrially produced by calcining pure silica at temperatures around 1,500 °C in a rotary kiln. This process leads to a recrystallization of silica, expanding the lattice structure and reducing density. Consequently, air inclusions are formed, giving a high degree of whiteness and specific optical properties. The production process includes washing, grading, and sieving the quartz, followed by drying, preheating, and finally calcination.
MORE INFORMATION?
For all questions regarding cristobalite, we are happy to assist you. Please do not hesitate to contact us – we will be glad to respond.






