Aluminum Hydroxide – An Indispensable High-Performance Filler
Aluminum Hydroxide is the key to innovative solutions in numerous industrial sectors. Whether used as a flame retardant, filler, or to improve coverage, its exceptional properties like high purity, chemical stability, and flame retardant effect make it indispensable. With applications spanning from the plastics and electronics industries to the chemical industry, aluminum hydroxide offers multiple possibilities to optimize your products.
Discover why aluminum hydroxide is an essential raw material for modern technologies and how it can bring your projects to the next level!
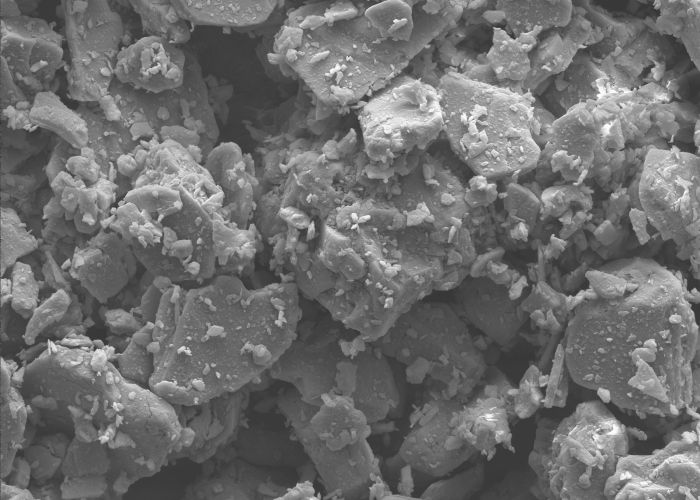
Chemical Properties
Aluminum Hydroxide (Al(OH)₃) is one of the most versatile chemical compounds in the industry and stands out for its specific physico-chemical properties. Its characteristic layered structure makes it an indispensable material for numerous applications.
Solubility and reactive behavior: Aluminum Hydroxide is practically insoluble in water (solubility at 20°C: approximately 1.5 mg/L). However, it shows amphoteric properties and reacts with both acids and bases.
Thermal behavior: Aluminum Hydroxide starts decomposing at approximately 180-200°C, releasing water. This endothermic process is the basis of its flame retardant action.
Coefficient of thermal expansion: The coefficient of thermal expansion of Aluminum Hydroxide is 15×10⁻⁶ K⁻¹ in the temperature range from 20°C to 300°C. This low expansion contributes to the thermal stability of Aluminum Hydroxide in composite materials.
Chemical resistance and inertness: Aluminum Hydroxide shows high chemical resistance in neutral and slightly alkaline environments. It is inert to many organic chemicals, making it ideal for applications in paints, coatings, and polymers.
Environmental compatibility: Aluminum Hydroxide is an eco-friendly material due to its low toxicity and biodegradability. It does not leave harmful byproducts during thermal decomposition, making it particularly suitable for environmentally sensitive applications such as flame retardants for textiles and plastics.
We are happy to support you in optimizing the full potential of Aluminum Hydroxide for your applications. Contact us for detailed advice and tailored solutions.
Technical Properties
- Low hardness: 3
- Density: 2,4 g/cm3
- High degree of whiteness (Y value >94)
- Coefficient of thermal expansion: 15*10-6K-1 (at T 20-300°C)
- Flame retardance
Advantages of Aluminum Hydroxide
- Cost reduction through the use of Aluminum Hydroxide as a filler
- Improvement of fire resistant properties in plastics and textiles
- High chemical stability and corrosion resistance
- High coverage and durability of paints and coatings
- Improvement of mechanical properties of materials
- Environmental compatibility due to recyclability and low toxicity
Aluminum Hydroxide from HPF: Products of the highest quality
Our products sold under the HPF brand are known as
HYDRAFIL®
Surface treatment of Aluminum Hydroxide
The surface treatment of Aluminum Hydroxide allows the formation of a hydrophobic or chemically resistant barrier. The use of surface treated Aluminum Hydroxide enables numerous advantages. These include reduced water absorption, better resistance to hot water, improved adhesion, optimized filling degrees and increased resistance to cathodic delamination.
In addition to technical advantages, there are also ecological and economic benefits due to the increased durability of materials.
Usage of Aluminum Hydroxide
Aluminum Hydroxide is an indispensable material in numerous industries: kitchen sinks, flame retardance, paints, coatings, or electronics. Aluminum Hydroxide offers flexible and high-performance solutions for your applications.
HYDRAFIL® is a high-performance filler based on Aluminum Hydroxide.

Efficient flame retardance in cables
Main areas of application:
- Flame retardant in plastics, textiles, carpets
- Product optimization in the cable industry
- Solid surface composites in PMMA
- SMC/BMC and latex
- Improvement of coverage in paints and coatings
- Heat dissipation in the electronics industrye
- Special applications in the chemical industry
- Hot water and chemical resistance in kitchen sinks
Aluminum Hydroxide is used as an efficient flame retardant in plastics, cables, and textiles and is a proven component of epoxy resins, SMC and BMC compounds, and latex products. It optimizes coverage and durability of paints and coatings and offers reliable solutions for heat dissipation in the electronics industry. In the chemical industry, it enables special applications that meet the highest standards.
Contact us for an optimal solution for your needs. Our experts are at your disposal.
Exceptional properties of Aluminum Hydroxide
Aluminum Hydroxide combines an impressive range of properties that makes it an indispensable material in numerous industries.
Fire Protection: Aluminum Hydroxide is valued as a highly effective flame retardant. At temperatures above 180°C, it releases water, mitigating flame development and minimizing smoke formation.
Resistance: Aluminum Hydroxide stands out for its chemical inertness and stability, making it ideal for use in demanding environments.
Low hardness: It is easily workable and allows seamless integration into mixtures without causing abrasiveness or stress on materials.
High coverage: The high coverage improves the optical properties of paints and coatings, ensuring uniform and durable results.
Umweltfreundlich: Durch seine nicht-toxischen Eigenschaften ist es sicher und umweltfreundlich, ideal für Anwendungen, bei denen Nachhaltigkeit entscheidend ist.
Environmental compatibility: Thanks to its non-toxic properties, it is safe and eco-friendly, ideal for applications where sustainability is essential.
High purity: The high purity offers an excellent basis for high-tech applications where maximum performance and precision are required, from electronics to the chemical industry.
Our expertise helps you make the most of Aluminum Hydroxide for your specific needs. Contact us!
Information on the production of Aluminum Hydroxide
The industrial production of Aluminum Hydroxide is achieved synthetically through chemical processing of bauxite, a rock rich in aluminum.
The process begins with the digestion of crushed bauxite. Subsequently, with the addition of seed crystals, Aluminum Hydroxide (Al(OH)₃) precipitates, which, after further processing steps, presents itself in the form of pure powder. Surface modifications such as surface treatment optimize the material's properties for specific applications.
Aluminum Hydroxide particles are distinguished by their high purity and specific characteristics, such as flame retardant effect, high degree of whiteness for optically pure final products, and low abrasiveness (low hardness), making Aluminum Hydroxide ideal for sensitive applications such as textiles or coatings.
Aluminum Hydroxide is widely used in various industrial sectors such as plastics, electronics, paints, and coatings industries.
Historical context of Aluminum Hydroxide
The history and industrial importance of Aluminum Hydroxide are closely linked to the development of the Bayer process. This process was developed by Carl Josef Bayer in 1888 and forms the basis for large-scale production of Aluminum Hydroxide.
Early industrial applications focused on aluminum production. Over time, however, the potential of Aluminum Hydroxide as a flame retardant, filler, and component in paints and coatings was recognized. Technological advances in the 20th century led to improvements in production and application, particularly through increased purity and surface modifications such as surface treatment.
The discovery of the thermal decomposition of Aluminum Hydroxide and its flame retardant properties significantly expanded its use in modern industrial processes. Today, Aluminum Hydroxide is an indispensable material in high-tech and sustainable technologies.
FAQ
What properties does Aluminum Hydroxide have?
Aluminum Hydroxide stands out for its low hardness (Mohs 3), high density (2.4 g/cm³), and high degree of whiteness (Y value >94). It has a coefficient of thermal expansion of 15 × 10⁻⁶ K⁻¹ in the temperature range from 20°C to 300°C. Additionally, it is flame retardant and offers multiple usage possibilities in various industries.
Where is Aluminum Hydroxide used?
- Plastics and textiles: As flame retardant and filler.
- Paints and coatings: To improve coverage and durability.
- Electronics industry: For heat dissipation in components.
- Cables: For fire protection and mechanical stability.
- Pharmaceuticals: As antacid and adjuvant in vaccines.
- Chemical industry: For special applications such as catalysts or absorbents.
How does Aluminum Hydroxide react?
- Improved fire resistance properties in plastics and textiles
- High chemical stability and corrosion resistance
- High coverage and durability for paints and coatings
- Improvement of mechanical properties of materials
- Environmental compatibility due to recyclability and low toxicity
How is Aluminum Hydroxide produced?
The industrial production of Aluminum Hydroxide is achieved synthetically through chemical processing of bauxite, a rock rich in aluminum. The process begins with the digestion of crushed bauxite. Subsequently, with the addition of seed crystals, Aluminum Hydroxide (Al(OH)₃) precipitates, which, after further processing steps, presents itself in the form of pure powder. Surface modifications such as surface treatment optimize the material's properties for specific applications.
More Information?
For any questions regarding Aluminum Hydroxide, we are happy to assist. Please do not hesitate to contact us – we will be happy to answer.






